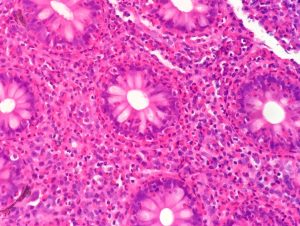
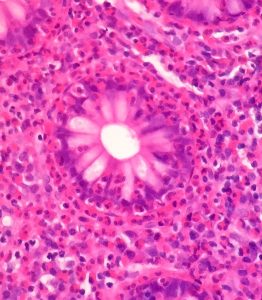
Answers to Questions Posted after Year 5 UGI Pathology Lecture
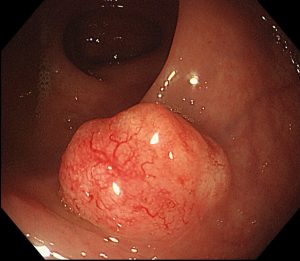
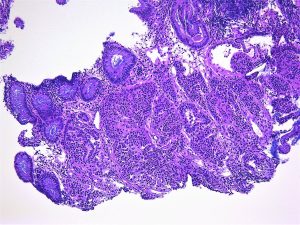
what are the key distinguishing factors that determine if a histological sample is dysplastic or cancer?
Invasion through the baseman membrane into the underlying connective tissue ( in the stomach that is into the lamina propria).
what causes squamous cell carcinoma? (ie you referenced it occurs more commonly in developing countries).
Smoking and/ or alcohol are important. HPV infection also has a role.
I think I missed it but what is CLO-IM?
Columnar Line Oesophagus- Intestinal Metaplasia
Is there such thing as chronic oesophagitis? Or does the inflammation just progress to Barrett’s over time?
These is, for example, with chronic gastro-oesophageal reflux. This will increase the risk of metaplasia
Are there any practical tests for determining cag-A status of H. pylori infections and are these tests used clinically at all?
The tests exist but are not used in routine clinical practice
How common is shock due to ulcer haemorrhage?
It depends how sever the haemorrhage is. Most bleeding from ulcers do not produce shock.
is there a genetic component? i.e. Japanese man migrating to England? (if that makes sense)
There is, and you give a good example, but environmental factors are also important. For example, even when people move they may take their diet with them.
why is there a high incidence in specifically in japan?
It is not only Japan that has a very high incidence: South Korea and Mongolia have even higher incidences. There are environmental as well as genetic factors but it is known that chronic gastritis with intesitnal metaplasia is commoner and more severe in these areas.
Can intestinal gastric cancer progress to diffuse?
I don’t think so but I know nothing published about this. Mixed intestinal and diffuse cancers are not uncommon.
are giardia and Whipple’s disease also associated with immunosuppression?
They are (as are almost all infections).