A simple guide to solar fuels 2 – Bringing to market
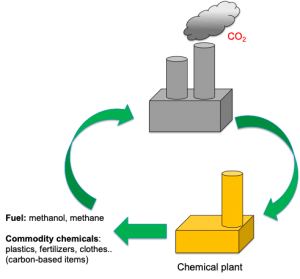
Last time we saw the potential solar fuels have in producing clean green hydrogen. But it turns out solar energy can also be used to convert CO2 and methane, potent greenhouse gasses, into high-value products for the production of fertilizers, plastics or even pharmaceuticals. In this post we find out how!
By Dr Miriam Regue and Dr Minsu Park, Research Associates in the Department of Chemical Engineering.
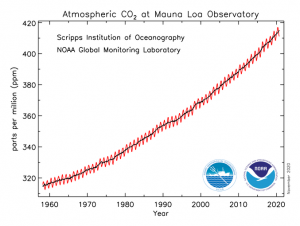
Solar fuels have the potential to not only help us reach net zero carbon emissions, but play an important role in the making of new carbon-based products following a sustainable circular economy approach (Fig. 1).
However, despite the great promise and potential of solar fuels, they are still far from being commercialised. But why? What is hindering the deployment of solar fuels, and what limitations are we facing?
It is both hard and challenging to give just one simple answer to this question. Remember from our first post that we said certain materials can absorb the energy from sunlight and transform it into electrical current? Well at the moment, the ideal light absorber materials that would lead the transition to a solar fuel-based society are still being researched. In fact, the “ideal” light absorber must fulfil several requirements. For one, it must absorb the widest possible range of the solar spectrum, so we can make the most of the solar energy to use it for the production of solar fuels. Secondly it must be made of elements with a plentiful supply on the Earth’s crust.
To understand the first requirement, we must consider the solar spectrum. It is composed of three different bands; infrared, visible and ultraviolet radiation. Infrared and visible radiation are the majority bands, accounting for 52 – 55 % and 42 – 43 % of the spectrum respectively, whereas ultraviolet radiation represents only 3 to 5 %. To absorb the complete range of the solar spectrum is still a challenging process within the research community. Unfortunately, the infrared radiation – the largest band in the solar spectrum – is not very energetic which makes it very difficult for activating the light absorber material and trigger the production of solar fuels. Therefore, light absorber materials can mainly be activated by either visible or ultraviolet radiation.But, considering the largest portion of visible radiation in the solar spectrum, the “optimum” light absorber material should be particularly good at absorbing light from the visible range (400 – 700 nm). Each material has a specific and unique absorption range – called band gap energy. The band gap energy and the absorption energy are indirectly related. The highest the absorption wavelength, the lowest the band gap energy. It turns out that the optimal band gap values that correspond to the visible wavelengths of the solar spectrum range from 1.9 to 3.1 eV.
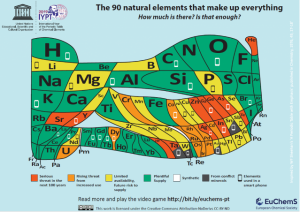
The second requirement, that our light absorber material must be made of elements with a plentiful supply on the Earth’s crust, can be solved by choosing a green-coloured elements of The Element scarcity periodic table (Fig. 2). Haematite (α-Fe2O3), which is one of the renowned materials for solar water splitting, is a promising light absorber candidate: It has a band gap energy of 2.1 eV, meaning that it can absorb near the range of 590 nm and it is made of large abundant elements, iron (Fe) and oxygen (O). This makes it fulfil both our requirements!
Ultimately, the efficiency of the process also depends on several inherent features of the light absorber material, such as their energetic properties and their ability to convert the absorbed solar energy to the desired solar fuel. In fact, these are the most challenging features to control and as mostly agreed by the research community, probably the limiting step of the solar fuel production. Finding the proper material able to meet all these requirements at once is a cumbersome process and requires the collaboration of scientists and engineers from different fields of expertise such as theoreticians and experimentalists. In fact, although Fe2O3 (haematite) fulfils the first and second requirements (made of abundant elements and suitable light absorbtion), its inherent properties are still not good enough to produce solar fuels in an efficient manner.
But if existing materials such as Fe2O3 (haematite) or TiO2 are not good enough for solar fuels, how can we improve them? How can we find novel materials with more suitable properties? We will find out in our next outing.