Written by Khansa Hussain
Edited by Caroline Anderson
Last November we had the pleasure to welcome Dr Joseph Boyle from Imperial College London to our IRD Journal Club. He nominated his publication “Activating Transcription Factor 1 Directs Mhem Atheroprotective Macrophages Through Coordinated Iron Handling and Foam Cell Protection” to introduce and discuss with us his interesting finding of identifying a unique state of macrophages (Mhem) within intraplaque hemorrhage (IPH). IPH is a common feature of atherosclerotic plaque damage which involves the rupture of neovessels causing blood to escape into the surrounding tissues. This leads to cholesterol and heme/iron loading. The monocytes that enter the plaques and differentiate to macrophages to clear hemorrhage-related iron or lipid are known as Mhem macrophages to discriminate them from the classic lipid-laden macrophages (foam cells).
Previously Dr Boyle has shown that heme induces a set of Mhem specific genes distinct from those that contribute to M1, M2, or Mox cell differentiation, which act to suppress HLA-DR and increase surface CD163 expression. In the paper they used microarray analysis of human blood-derived monocytes stimulated with heme to identify the mechanism that regulates this functional specialization. Activating transcription factor 1(ATF-1) was one of the most upregulated genes immediately after stimulation, but subsequently there was upregulation of effectors genes such as heme oxygenase 1 (HO-1). Importantly while upregulation of ATF1 mRNA was rapid and transient, ATF-1 and pATF-1 protein were upregulated at the same time as HO-1 transcripts. siRNA oligonucleotides that suppressed ATF-1 or p-ATF-1 caused downregulation of both HO-1 mRNA and protein expression in response to heme. Conversely, transfection with an ATF-1–expressing plasmid increased p-ATF-1 and HO-1 mRNA and protein production. D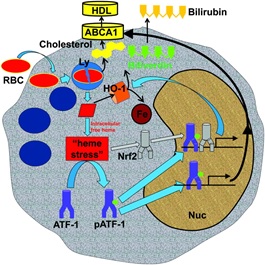 r Boyle’s team conducted further transcriptional analysis that also highlighted the transcription factor liver X receptor beta (LXR-β), a “master regulator” of lipid metabolism. They therefore hypothesize that ATF-1 directs the production of both LXR-β and HO-1, and expression of a network of genes responsible for lipid and iron handling.
r Boyle’s team conducted further transcriptional analysis that also highlighted the transcription factor liver X receptor beta (LXR-β), a “master regulator” of lipid metabolism. They therefore hypothesize that ATF-1 directs the production of both LXR-β and HO-1, and expression of a network of genes responsible for lipid and iron handling.
Lastly they examined serial sections of human plaques to see if they could discriminate Mhem macrophages from foam cells. Mhem were smaller than foam cells, suggesting that they were resistant to becoming foam cells. They also found colocalization of p-ATF-1 with HO-1 and ABCA1 (adenosine-triphosphate-binding-cassette-transporter (ABC) proteins-A1) in Mhem cells but not in foam cells. ABCA1 is a key cholesterol exporter for HDL, indicating that the Heme/ATF-1 pathway drives lipid export and protects cells from becoming foam cells.
We conclude from this interesting paper that in IPH macrophages differentiation is a key pathophysiological mechanism dependent on specific signaling with the plaque (summarized in the attached cartoon taken from the paper). Mhem-specific gene expression could explain the functional mechanisms of how cells handle iron and lipid and become atheroprotective macrophages.
Read the full article here:
http://dx.doi.org/10.1161/CIRCRESAHA.111.247577