Written by William Branchett
Edited by Judith Secklehner and Caroline Anderson
The immune system has a huge capacity for destruction which, if unrestrained, can cause collateral damage to host tissue. Immune responses must therefore have ‘off’ switches to limit their magnitude and avoid inflammatory disease. Conversely, pathogens and tumours can hijack these mechanisms of immune regulation to favour their own survival. Understanding of immune regulation may therefore allow the design of drugs to manipulate overly exuberant or insufficient immune responses.
Transforming growth factor β (TGF-β) is a multi-functional cytokine that can dampen immune responses, in part by suppressing the pro-inflammatory activity of effector CD4+ T cells (Teffs). Previously, experiments from the labs of Fiona Powrie and Richard Flavell demonstrated that both regulatory T cells (Tregs) and TGF-β are required to prevent the pathogenic action of Teffs in a mouse model of colitis1. Unexpectedly, they also found that Tregs were not an essential source of TGF-β for this process1. So if not required to produce TGF-β, how do Tregs fit into the picture?
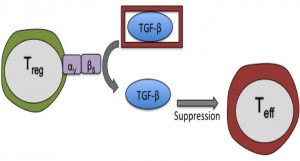
TGF-β is unusual among cytokines, as it must be released from a latent precursor complex to perform its biological functions. Among other mechanisms, latent TGF-β can be activated by interaction with certain integrins on immune and stromal cells. In their recent paper in Immunity, Worthington, Travis and colleagues highlight integrin-dependent TGF-β activation as a major role of Tregs in preventing T cell-driven inflammation2. The authors showed that integrin αVβ8 is highly expressed by Tregs in mice and humans, and allows Tregs to proficiently activate latent TGF-β. Expression of αVβ8 on Tregs was necessary to limit the severity of mouse models of colitis and skin inflammation, most likely by providing active TGF-β to pathogenic Teffs. Interestingly, mice lacking αVβ8 on their Tregs showed no signs of spontaneous inflammatory disease- suggesting that TGF-β activation by this mechanism is required during active inflammation but not at homeostasis.
There was much interest in this novel regulatory mechanism from the Journal Club group; particularly since production, rather than activation, of TGF-β is traditionally associated with the suppressive function of Tregs. As this story unfolds further, it will be exciting to hear more about the role of αVβ8 on Tregs in human disease and whether drugs targeting αVβ8 can selectively modulate TGF-β activity in the vicinity of inflammatory diseases and tumours.
Read the full paper here: http://www.cell.com/immunity/fulltext/S1074-7613%2815%2900172-7
References:
- Fahlén et al., Journal of Experimental Medicine. Vol. 201 no. 5 737-746
- Worthington et al., Immunity. Vol. 42 no. 5 903-915