NHLI researchers Róisín Mongey and Dr Sally Kim provide an insight into developing a new tool – the AIR model – for lung research and drug development.
Lung diseases represent a significant global health burden costing the NHS upwards of £1 billion annually. A hallmark of chronic and acute adult lung diseases such as Chronic Obstructive Pulmonary Disease (COPD), Idiopathic Pulmonary Fibrosis (IPF) and COVID-19, is lung damage. The lungs are usually capable of repairing damage but in some cases, this does not happen or the repair process goes awry for example going into overdrive and causing more damage. The result of this lack of repair or abnormal repair is persistent tissue damage and declining lung function.
There are almost no treatments available to repair the lung damage in these diseases. A bold, new approach to identify novel lung repair treatments for these diseases is needed. Unfortunately, there are several roadblocks to the development of curative treatments, the primary one being that we don’t fully understand how repair happens in the healthy lung under normal circumstances. The bottom line is that unless we can figure this out, it is unlikely that we will be able to develop successful new repair treatments.
How exactly do we develop new treatments?
For obvious reasons, studying the mechanisms of repair and regeneration in the human lung is challenging given their essential nature for life. The use of experimental models which properly replicate the lung environment is therefore the next best thing and the development of such models is an essential area of focus in the lung repair field. An ideal biologically relevant experimental model needs to tick a number of important boxes:
- having relevant cell types,
- having a functional network of blood vessels and maintaining the tissue architecture,
- being reproducible, high throughput and low cost.
In addition, a model should accurately replicate the disease/damage that it is being used to study. In the lung, the type of damage that occurs can differ depending on the disease, however one common feature across conditions is the combination of injured regions next to relatively healthy tissue, particularly in the early stages of disease. How cells respond to lung damage in injured and uninjured regions is likely to be different and we believe that replicating this in an experimental model for the study of lung damage is important. Often meeting all of these criteria is not possible however we aimed to tick as many of these boxes as possible when developing our model for the study of lung injury and repair: The Acid Injury and Repair (AIR) model.
The journey to developing a model
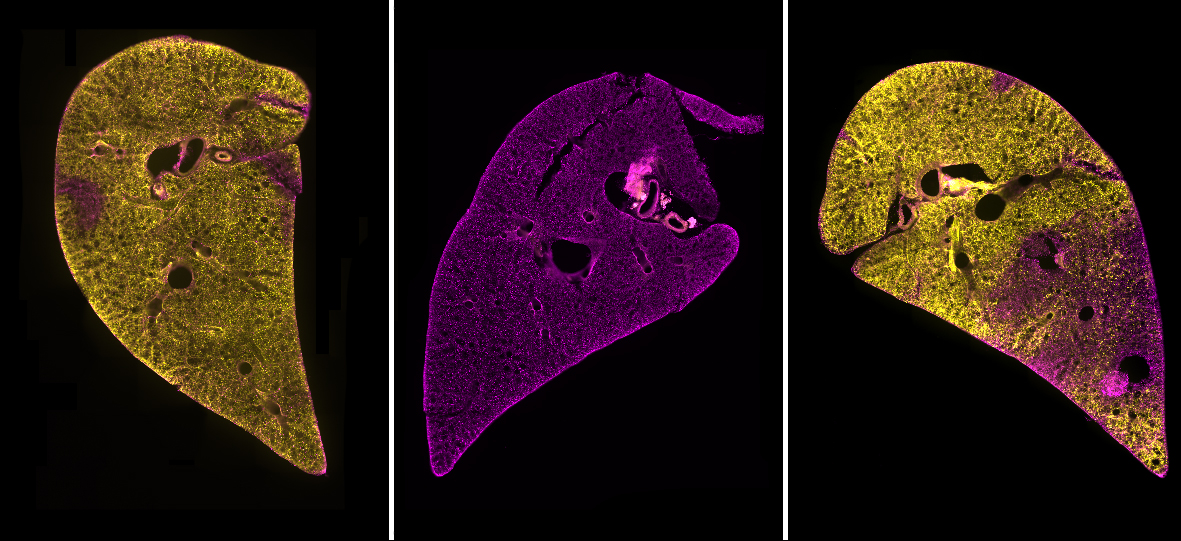
We began the process of developing a model by searching for a system that included as many of the “ideal” criteria as possible. To have a model that contained all of the different cell types combined in the usual structure of the lungs, we chose to use precision cut lung slices (PCLS). These are thin sections of lung tissue that can be kept alive through tissue culture methods for a number of days. PCLS are easy to produce and do not require the use of live mouse lines or complex bioengineered models.
To recreate the combination of damaged and healthy tissue often seen in disease we began by trialling different methods of applying injury to a restricted region of the PCLS. Our initial trials included applying a physical scratch, strips of paper soaked in hydrochloric acid and hydrochloric acid diluted in a viscous gel. None of these options however were successful in generating an isolated region of damage. We decided to stick with acid because inhalation of stomach acid (gastric aspiration injury) frequently causes lung damage in patients with lung disease as well as in ICU patients who are on ventilators.
We next decided to trial restricting the area of injury using a method for isolating a region of cells in a cell culture dish. To do this we took a small plastic cloning cylinder and coated one edge with silicone grease. The cylinder was then placed grease side down on the PCLS to create a sealed region to which we could apply our HCl injury. We also added the HCl in a viscous gel as a further measure to prevent the acid leaking outside the cylinder. The acid was only briefly applied to the tissue and was then removed to prevent further damage.
Stem cells offer new clues
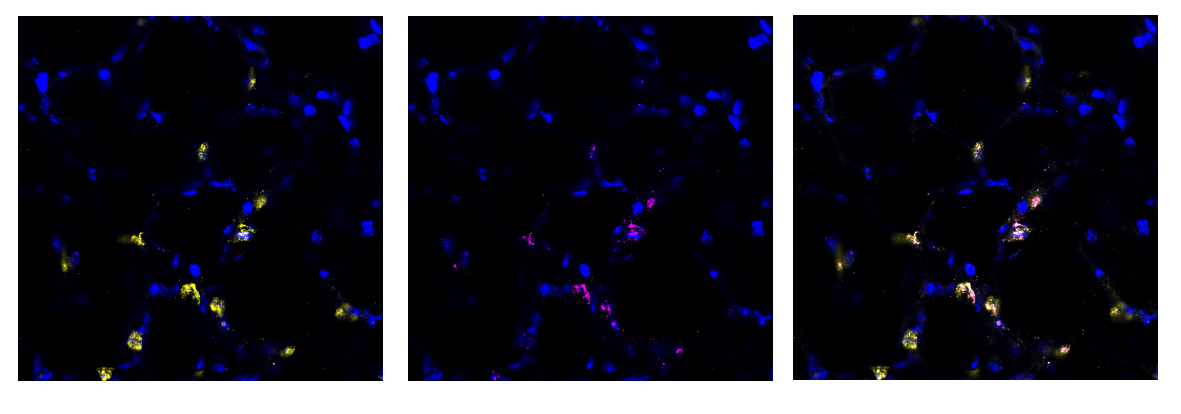
We found this method to be highly successful at generating a region of damaged tissue while retaining healthy tissue in the rest of the lung slice. Using special fluorescent stains that recognise either live cells or dead cells we could clearly distinguish the injured and uninjured areas of the tissue down the microscope. Having established a method of injuring a region of lung tissue we then went on to examine how cells in the injured and uninjured areas responded to the acid. We found a huge increase in stem cells in the injured area of tissue. Stem cells are known to be important for repairing and regenerating injury in the lungs and many other organs in the body. This was an exciting finding and shows that the AIR model will be really useful for scientists to study lung repair.
What will this model help us to achieve
Another key advantage of our model is that it has potential to be used as a semi high-throughput screening tool for new drugs or repair treatments. We have developed methods to fluorescently label many specific cell types in PCLS that are important for studying lung injury and repair (including the stem cells mentioned above). Therefore, we can visualise repair and regeneration by analysing changes in lung biology, including the number, size and morphology (shape) of specific cells in the acid-injured and uninjured (healthy) lung tissue. To adapt the AIR model for use as a screening tool, we used a widefield microscope with a structured illumination system that removes out of focus light while enhancing the image resolution. We also used a specifically designed cell counting macro for data to be generated in an automated manner. Both of these adaptations mean that multiple PCLS can be investigated simultaneously.
As proof of concept, we treated acid-injured PCLS with drugs and we were then able to observe and quantify changes that resulted from the injury. This demonstrated that our model could be used as a screening tool to identify new drugs for lung tissue repair.
The AIR model will be an important tool that can be used to bridge the gap between cell culture and animal studies and therefore ultimately reduce animal use in research. There is also the potential for this model to be adapted for use in other organs and we anticipate that it will be a versatile tool in life sciences research.
Ms Róisín Mongey has just completed her PhD and is a research assistant in the Lung Development and Disease group at the National Heart and Lung Institute (NHLI).
Dr Sally Kim is a research associate in the Lung Development and Disease group.
One comment for “A breath of fresh ‘AIR’ in the study of lung repair and regeneration”